Abstract
Background. Busulfan plus Cyclophosphamide (BuCy) is the traditional myeloablative conditioning (MAC) regimen pre allogeneic stem cell transplantation (SCT) for acute myelogenous leukemia (AML). The Thiotepa, Busulfan, Fludarabine (TBF) protocol was initially developed for cord blood SCT and subsequently employed in haploidentical SCT; however, there is limited data assessing the TBF regimen in SCT from sibling (MSD) and unrelated donor (URD) for AML and no comparison between the two preparative protocols has been performed.
Patients and methods. We analyzed transplantation outcomes of 2523 adult patients (pts) with AML in remission (CR1, CR2+) aged 18-50 years that underwent a first SCT from MSD or URD between 2007 and 2015 and reported to the ALWP of the EBMT. One hundred and three patients received TBF and 2370 pts received BuCy. Pts who received oral busulfan, manipulated grafts, or transplant from <=8/10 HLA-matched donor were excluded. All pts received MAC regimen (defined by Busulfan dose>=9.6 mg/kg). As compared to BuCy, the TBF group included significantly older pts, who were transplanted more recently, had longer interval from diagnosis to transplant, and were more likely to have received BM as stem cell source and URD as donor type. In order to minimize the bias due to differences in pts characteristics, a 1:3 pair-matched analysis was performed. One hundred and forty-six pts receiving TBF were compared with 438 pts receiving BuCy; matching factors included age, year of SCT, disease status at SCT, interval from diagnosis to SCT, donor type, CMV serostatus of both the donor and patient, stem cell source, GVHD prophylaxis and use of anti-thymocyte globulin.
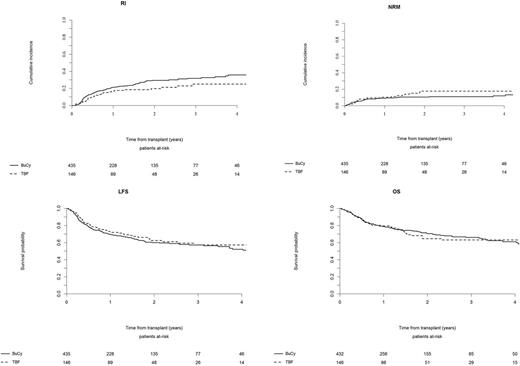
Results. With a median follow-up of 22 months, incidence of grade II-IV and III-IV aGVHD after TBF vs BuCy was 29% vs 23% (p=0.23) and 15% vs 8% (p=0.04), respectively. The 5-year cumulative incidence of cGVHD and severe cGVHD was 36% vs 43% (p=0.66) and 16% vs 22% (p=0.72) for TBF vs BuCy, respectively. The 1-year and 5-year non-relapse mortality (NRM) rates were 10% and 22% for TBF vs 9% and 13% for BuCy, respectively (p=0.36). The 1-year and 5-year relapse incidence (RI) rates were 17% and 25% for TBF vs 21% and 37% for BuCy, respectively (p=0.32). No significant difference was observed in leukemia-free survival (LFS), being the 1-year and 5-year LFS rates 73% and 53% for TBF vs 70% and 50% for BuCy, respectively (p=0.56). Overall survival (OS) was also similar, being 80% and 54% for TBF vs 79% and 59% for BuCy, respectively (p=0.88). The composite endpoint graft-vs-host disease-free, relapse-free survival (GRFS) at 5 years was 48% for TBF and 40% for BuCy (p=0.48). Notably, when the analysis was restricted to pts in CR1, a significant difference in RI rate was observed (1-year and 5-year RI: 10% and 18% for TBF vs 20% and 38% for BuCy, respectively, p=0.003) leading to a trend for better LFS for TBF as compared to BuCy (1-year and 5-year LFS: 78% and 57% for TBF vs 72% and 50% for BuCy, respectively, p=0.07), with no difference in OS. When examining separately pts receiving SCT from MSD or URD, no significant difference was observed between TBF and BuCy regimens in terms of transplant outcome. In multivariate analysis, TBF was associated with lower RI compared to BuCy (HR 0.6, p=0.02), while no difference was observed in terms of NRM, LFS, OS and GRFS (HR 0.8, p=0.12 for GRFS). TBF was also associated with lower incidence of cGVHD (HR 0.7, p=0.04) and a trend for lower incidence of severe cGVHD (HR 0.6, p=0.1); on the contrary we observed a trend for higher incidence of grade III-IV aGVHD following TBF as compared to BuCy (HR 1.79, p=0.06).
Conclusions. These results suggest that TBF is a valid myeloablative conditioning pre MSD or URD SCT for AML pts < 50 years of age and in remission, with similar transplantation outcome as compared to the traditional BuCy preparative regimen. These data may serve as the scientific background for a well designed randomized two arm study comparing TBF to BuCy as MAC pre SCT in AML pts below the age of 50 years in CR.
Savani: Jazz Pharmaceuticals: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.